Atom
The atom is the basic, smallest unit of matter. Therefore it is indivisible, uncuttable, or cannot be divided any further, as the definition of the Greek word "atomos." As shown in the picture above, the atom is composed of electrons, protons, and neutrons. The atomic nucleus consists of protons and neutrons, and it is surrounded by negatively charged ions, called electrons. The electrons of an atom are bound to the nucleus by the electromagnetic force.
An atom is classified according to the number of protons and neutrons in its nucleus: the number of protons determines the chemical element, and the number of neutrons determines the isotope of the element.
A group of atoms can be bound to each other, forming a molecule.
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge.
A molecule may consist of atoms of a single chemical element, as with oxygen (O2), or of different elements, as with water (H2O). Atoms and complexes connected by non-covalent bonds such as hydrogen bonds or ionic bonds are generally not considered single molecules.
Chemical Bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are "strong bonds" such as covalent or ionic bonds and "weak bonds" such as hydrogen bonding.
Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons orbiting the nucleus and the positively charged protons in the nucleus attract each other. Also, an electron positioned between two nuclei will be attracted to both of them. Thus, the most stable configuration of nuclei and electrons is one in which the electrons spend more time between nuclei, than anywhere else in space. These electrons cause the nuclei to be attracted to each other, and this attraction results in the bond. However, this assembly cannot collapse to a size dictated by the volumes of these individual particles. Due to the matter wave nature of electrons and their smaller mass, they occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves.
Types of Chemical Bonds
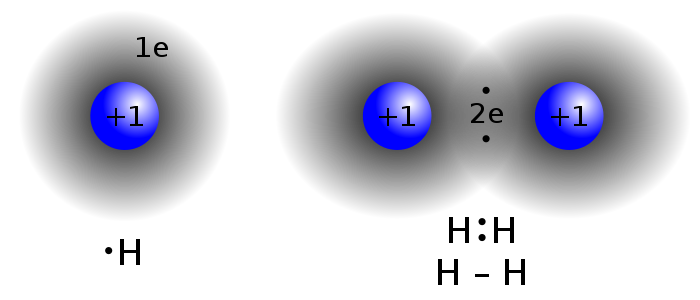
Covalent Bond
a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms, and other covalent bonds. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
Two hydrogen atoms sharing the same electrons.
Ionic Bond
In ionic bonding, electrons are completely transferred from one atom to another. In the process of either losing or gaining negatively charged electrons, the reacting atoms form ions. The oppositely charged ions are attracted to each other by electrostatic forces, which are the basis of the ionic bond.
During the reaction of sodium with chlorine, sodium (left) loses its one valence electron to chlorine (right), resulting in:
a positively charged sodium ion (left) and a negatively charged chlorine ion (right).
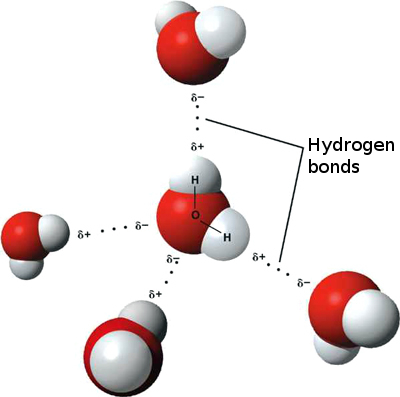
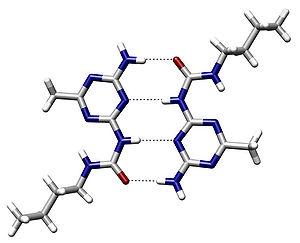
Hydrogen Bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen, our fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond. These bonds can occur between molecules (intermolecularly), or within different parts of a single molecule (intramolecularly). The hydrogen bond (5 to 30 kJ/mole) is stronger than a van der Waals relation, but weaker than covalent or ionic bonds. This type of bond occurs in both inorganic molecules such as water and organic molecules such as DNA.
An example of intermolecular hydrogen bonding.



No comments:
Post a Comment